
Belay Vantage™
MGMT promoter methylation in CSF
An advanced approach to determining MGMT promoter methylation status
Belay Vantage uses quantitative polymerase chain reaction to evaluate MGMT promoter methylation in CSF of individuals with known or suspected CNS tumors.
Why choose Belay Vantage?
- Analysis in CSF sample is less invasive than brain biopsy
- MGMT promoter methylation testing is recommended in all high-grade gliomas1
- Serves as a predictive biomarker for temozolomide (TMZ) response in patients with IDH1-wild-type malignant gliomas2
- Prognostic in gliomas with IDH1 mutation treated with combined TMZ chemo-irradiation and associated with extended progression-free survival2
- Median survival increases 50% in glioblastoma patients when treated with TMZ if MGMT promoter is methylated3
1. Horbinski C, Ligon KL, Brastianos P, et al. The medical necessity of advanced molecular testing in the diagnosis and treatment of brain tumor patients. Neuro Oncol. 2019 Dec 17;21(12):1498-1508. doi: 10.1093/neuonc/noz119. PMID: 31276167; PMCID: PMC6917404
2. Wick W, Meisner C, Hentschel B, et al. Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology. 2013;81(17):1515-1522. doi:10.1212/WNL.0b013e3182a95680
3. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073-1113.doi:10.1093/ neuonc/noaa106
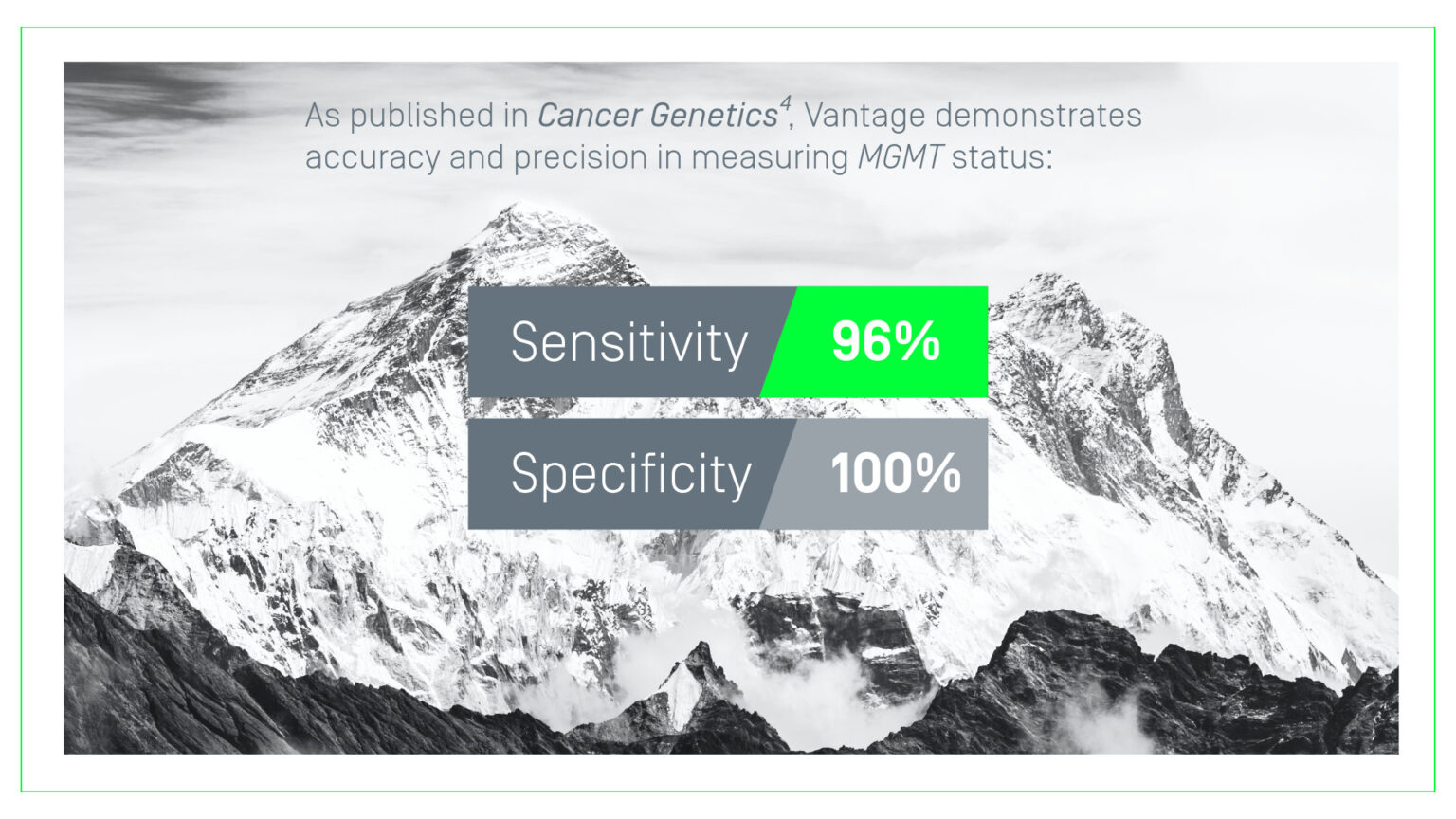
4. Schilter KF, Nie Q, Adams JN, Jagadish R, Acevedo A, Larson A, Vo SA, Domagala BA, Hernandez KM, Douville C, Wang Y, Coe B, Bettegowda C, Reddi HV. Analytical validation of the Belay Vantage™ assay for evaluation of MGMT promoter methylation using enzymatically converted tumorDNA from cerebrospinal fluid (CSF). Cancer Genet. 2025 Jun;294-295:94-98. doi: 10.1016/j.cancergen.2025.04.001. Epub 2025 Apr 5. PMID: 40250264.